
What is Diabetic Ketoacidosis (DKA)?
Diabetic ketoacidosis (DKA) is a problem that occurs in people with diabetes. It occurs when the body cannot use sugar (glucose) as a fuel source because there is no insulin or not enough insulin, and fat is used for fuel instead.
Key symptoms can include:
- Rapid and deep breathing
- Dry skin and mouth
- Flushed face
- Fruity smelling breath
- Nausea and vomiting
- Stomach pain
Other symptoms include:
- Abdominal pain
- Breathing difficulty while lying down
- Decreased appetite
- Decreased consciousness
- Dulled senses that may worsen to a coma
- Fatigue
- Frequent urination or thirst that lasts for a day or more
- Headache
- Muscle stiffness or aches
- Shortness of breath
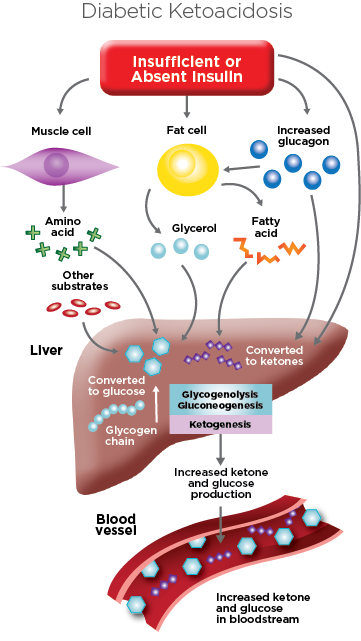
People with type 1 diabetes may be at risk when they do not have enough insulin, a hormone the body uses to break down sugar (glucose) in the blood for energy. When the body senses glucose is not available, fat is broken down instead. As fats are broken down, acids called ketones build up in the blood and urine. Ketones are poisonous in high levels and this condition is called ketoacidosis.
Blood glucose levels rise (usually higher than 300 mg/dL) because the liver makes glucose to try to combat the problem. However the cells cannot pull in that glucose without insulin.
Diabetic ketoacidosis (DKA) may be the first sign of type 1 diabetes in people who do not yet have other symptoms. It can also occur in someone who has already been diagnosed with type 1 diabetes. Infection, injury, a serious illness, or surgery can lead to diabetic ketoacidosis (DKA) in people with type 1 diabetes. Ketoacidosis can also occur in people with diabetes when they miss their insulin treatments.
People with type 2 diabetes can develop ketoacidosis, but it is rare. It is usually triggered by a severe illness. Hispanic and African-American people are more likely to have ketoacidosis as a complication of type 2 diabetes.
What is Beta-Hydroxybutyrate (BHB)?
During ketosis, Beta-Hydroxybutyrate (BHB) levels may increase more than levels of acetone and acetoacetate, clearly indicating the patient’s trend in metabolic status.
BHB demonstrates excellent stability, making it an excellent indicator of clinically relevant ketosis and ketoacidosis.
BHB is the main ketone body produced during ketosis. The pie chart shows a typical example of the keytone bodies of someone with severe diabetic ketoacidosis (DKA).
The Stanbio Chemistry Beta-Hydroxybutyrate LiquiColor® Reagent
The Stanbio Chemistry LiquiColor® BHB reagent is an enzymatic assay used by many healthcare systems in the United States.
BHB may be run on most chemistry analyzer systems with an open channel capability.
Key Features and Benefits:
- Quantitative results
- Enzymatic test
- Excellent sensitivity and precision
- Can be automated on a chemistry analyzer
- Uses serum or plasma
- Liquid, ready to use reagents
BHB Frequently Asked Questions
The Stanbio LiquiColor® BHB test can be used to determine the presence and levels of BHB, the predominant ketone body produced during ketoacidosis.
When the body breaks down fats in response to a low supply of energy (glucose) it produces three ketone bodies; Acetone, Acetoacetate and BHB.
Ketones (Acetone and Acetoacetate) can be tested or monitored in either urine or blood. Many hospitals still use the nitroprusside test for detecting and monitoring ketosis and ketoacidosis.
This nitroprusside urine and serum method produces a qualitative assessment of ketosis by detecting the ketones acetoacetate and acetone — it does not detect BHB.
This is significant because:
- BHB is not a ketone, it is a hydroxyacid.
- BHB demonstrates excellent stability, making it the most reliable indicator of clinically relevant ketosis and ketoacidosis.
- During ketosis, BHB levels increase more than levels of acetone and acetoacetate, clearly indicating the patients trend in metabolic status.
- Quantitative, objective results provide a better tool for differentiating metabolic acidosis and monitoring therapy.
Physicians use ketone body determinations in emergent or urgent medical situations such as diabetic ketoacidosis. Hospitals regularly measure ketone bodies using spectrophotometric methods. Note that the urine dip stick method for acetoacetate does not detect β-hydroxybutyric acid. Rapid measurements using urine dip sticks in hospitals may not be as accurate and may require confirmation by a reference lab.
- Ability to detect and quantify BHB
- The LiquiColor BHB test is specific for BHB, it does not react with the other ‘ketones’ in the sample
- The LiquiColor BHB test is quantitative, so it can be used to for diagnosis and monitoring (trend) of the patient’s status
- The LiquiColor BHB can be automated as an open channel test on most chemistry analyzers
Supporting Information
Title: BHB – Uses in the ICU Especially in the Setting of DKA
Presented by: Mark. H Oltermann MD
Location: JPS Physician Group, John Peter Smith Hospital, Fort Worth, Texas
Title: The Value Efficacy and Efficiency of β-Hydroxybutyrate
Presented by: James. H Nichols, PhD, DABCC, FACB
Location: The 2012 Clinical Lab Expo in Los Angeles
Title: Metabolic Disturbances that Lead to B-hydroxybutyrate and Ketone Bodies
Presented by: James. H Nichols, PhD, DABCC, FACB
Location: Pathology Grand Rounds at mid-west medical school
- Savage MW, Dhatariya KK, Kilvert A, Rayman G, Rees JA, Courtney CH, et al. Joint British Diabetes Societies guideline for the management of diabetic ketoacidosis. Diabet Med. May 2011;28(5):508-15. [Medline].
- Joint British Diabetes Societies Inpatient Care Group. The Management of Diabetic Ketoacidosis in Adults. March 2010. Available at http://www.diabetes.nhs.uk/document.php?o=1336. Accessed June 27, 2011.
- Wallace TM, Matthews DR. Recent advances in the monitoring and management of diabetic ketoacidosis. QJM. Dec 2004;97(12):773-80. [Medline].
- Timmons JA, Myer P, Maturen A, et al. Use of beta-hydroxybutyric acid levels in the emergency department. Am J Ther. May 1998;5(3):159-63. [Medline].
- Taboulet P, Haas L, Porcher R, et al: Urinary acetoacetate or capillary beta-hydroxybutyrate for the diagnosis of ketoacidosis in the Emergency Department setting. Eur J Emerg Med 2004; 11(5):251-258.
- Carragher FM, Bonham JR, & Smith JM: Pitfalls in the measurement of some intermediary metabolites.. Ann Clin Biochem 2003; 40(4):313-320.
- Arora S, Henderson SO, Long T, Menchine M. Diagnostic Accuracy of Point-of- Care Testing for Diabetic Ketoacidosis at Emergency-Department Triage: {beta}-Hydroxybutyrate versus the urine dipstick. Diabetes Care. Apr 2011;34(4):852-4. [Medline]. [Full Text].
- Arora S, Henderson SO, Long T, Menchine M. Diagnostic Accuracy of Point-of- Care Testing for Diabetic Ketoacidosis at Emergency-Department Triage: {beta}-Hydroxybutyrate versus the urine dipstick. Diabetes Care. Apr 2011;34(4):852-4. [Medline]. [Full Text].
- Menchine M, Probst MA, Agy C, Bach D, Arora S. Diagnostic accuracy of venousblood gas electrolytes for identifying diabetic ketoacidosis in the emergency department. Acad Emerg Med. 20 Oct;18(10):1105-8. doi: 10.1111/j.1553-2712.2011.01158.x. Epub 2011 Sep 26. PubMed PMID: 21951652.
- Laffel L. Ketone bodies: a review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab Res Rev. 1999 Nov-Dec;15(6):412- 26. Review. PubMed PMID: 10634967.
- Lone SW, Siddiqui EU, Muhammed F, Atta I, Ibrahim MN, Raza J. Frequency, clinical characteristics and outcome of diabetic ketoacidosis in children with type-1 diabetes at a tertiary care hospital. J Pak Med Assoc. 2010 Sep;60(9):725-9. PubMed PMID: 21381577.
- Goldstein DE, Little RR, Lorenz RA, Malone JI, Nathan DM, Peterson CM. Tests of glycemia in diabetes Technical review, Diabetes Care. 1995;18(6):896-909.
LEGAL DISCLAIMER : The material on this site is for informational purposes only. The content herein is not a substitute for the advice of qualified medical professionals. Only physicians and qualified medical professionals should be consulted for specific questions regarding medical conditions, testing and treatment. Stanbio Laboratory disclaims any liability for decisions made based on the information presented here or for the information provided on external Internet web sites. Stanbio Laboratory is not responsible and provides no warranty whatsoever for the accuracy, effectiveness, timeliness and suitability of any information or content obtained from third parties, including hyperlinks to or from third party websites. If you disagree with these terms and conditions (as they may be amended from time to time), or are dissatisfied with this website, your sole and exclusive remedy is to discontinue using this site.



